Nanocoulter Ⅰ Nanocoulter Single Particle Analyzer
—— A Whole "Core" Method for Detecting Extracellular Vesicle
Extracellular vesicles (EVs) refer to vesiculate corpuscle with double membrane structure that are shed from the cell membrane or secreted by cells. Its diameter is 40nm to 1000nm.
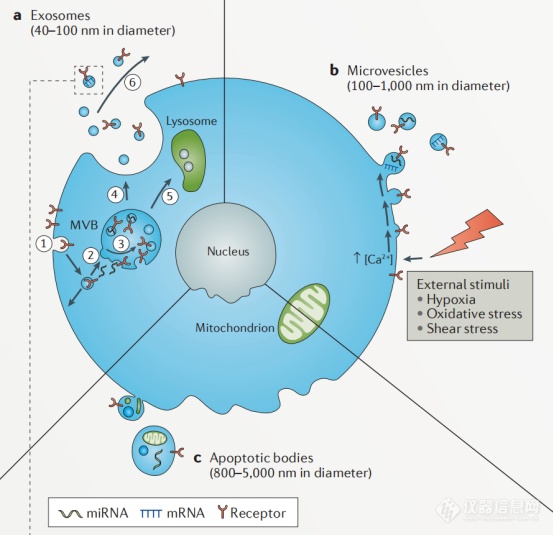
图1 细胞外囊泡的形成过程
Extracellular vesicles are mainly composed of microvesicles (MVs) and exosomes (Exs). Microvesicles are small vesicles shedding from cell membrane after cell activation, injury or apoptosis, with a diameter of about 100nm-1000nm. Exosomes are small membranous vesicles that are released by cell into the extracellular matrix, with a diameter of about 40-100nm (some said 30-150nm, however, is not yet determined). They are widely present in supernatant of cell culture and various body fluids (blood, lymph, saliva, urine, semen, milk). They carry a variety of proteins, lipids, DNA, mRNA and miRNA related to cell origin and participate in the processes of intercellular communication, cell migration, angiogenesis and immune regulation. Elevated levels of extracellular vesicles were found in diabetes, cardiovascular diseases, AIDS, chronic inflammatory diseases and cancer. Therefore, they are likely the diagn.
Compared with the popularity of exosome research (217 related papers were published in June alone in China), methods of the purification and extraction of exosomes were unsatisfactory. The current mainstream methods include ultracentrifugation (UC), density gradient centrifugation (DGC), immune capture, polymer precipitation (Precip), ultrafiltration (UF), size-exclusion chromatography (SEC), and microfluidic analysis chips. One extraction method is often unable to obtain sufficient purity and concentration of exosomes, and the applicable sample types of each method are different.
The International Society for Extracellular Vesicles (ISEV) proposed in 2014 that exosomes isolated need to be identified from three levels: (1) Identification of exosome surface markers by Western Blot (WB); (2) Identification of exosome morphology by transmission electron microscope (TEM); (3) Identification of exosome size by particle size analysis. Western Blotting and electron microscope are time-consuming, complex and expensive. It is difficult to perform WB and TEM for each exosome sample in practical research. Therefore, a quick and simple particle size analysis method is urgently needed to be used as the pioneer force for exosome identification.
Nanocoulter Ⅰ "core" method assisted extraction and purification research of exosomes
Nanocoulter Ⅰ single particle detection can be used for liquid biopsy and one sample test in only 5 – 10 min, and the test sample has the characteristics of zero pollution and recoverability, which meets the demand. At present, it has been widely used in the extraction and identification of extracellular vesicles (exosomes).

Fig.2 Appearance of Nanocoulter Ⅰ
Principle of Nanocoulter Ⅰ
Nanocoulter principle. The process of testing exosomes is to disperse them in the electrolyte. As they pass through the nanopore chip with a specific aperture, the exosomes will occupy the position of the same volume of electrolyte as they pass through the nanopore. The resistance between the two electrodes inside and outside the nanopore (the circuit designed by constant current) will change instantly and generate potential pulse. The intensity and number of pulse signal are proportional to the size and number of exosome particles passing through the nanopore. The information of particle size and number of exosome particles can be obtained by directly converting the pulse signal.

Fig. 3 Principle diagram of Nanocoulter Ⅰ

Fig. 4 Core technology of Nanocoulter Ⅰ
Performance parameters of Nanocoulter Ⅰ:
Particle size range of detection: 40nm-2μm
Dimensional measurement accuracy: 1nm
Concentration range: 1E+06~1E+12/mL
Sample size: 50μL
Repeatability error: < 10%
Accuracy error of particle size: < 10%
Accuracy error of concentration: < 20%
Rapid detection of particle size distribution and concentration of exosome samples:
With the development of research, more methods of isolation and extraction of exosomes have been developed, and there are also a variety of methods combining isolation and extraction. Although these methods seem bountiful, in fact the qualities varied, and most are even not satisfactory. Nanocoulter Ⅰ can be used to quickly distinguish the advantages and disadvantages of extraction methods by measuring the particle size of exosomes one by one and getting the real particle size concentration distribution of exosomes.

Fig. 5 Determination of particle size of exosomes by different isolation and extraction methods
Nanocoulter Ⅰ single particle detection can obtain the real distribution state of all particles, and quickly, accurately and firmly distinguish the qualities of extraction methods, thus greatly saving time and labor costs.
Nanocoulter Ⅰ is “fast”
The core component chip is measured by laser etching, with high nanopore consistency. All the benchmarking information has been loaded into the two-dimensional code in the detection card. Therefore, the instrument does not need re-calibration, benchmarking and other tedious pre-test steps, saving a lot of valuable time. One card goes with one code. You can start testing by scanning two-dimensional code and adding samples. The samples only exist in the sample well of the plug-in detection card, and the activity of exosomes is not affected during detection. The valuable exosome samples can be recovered for subsequent research after the test is completed.
.jpg)
Fig. 6 Nanocoulter Ⅰ detection card
Nanocoulter Ⅰ is “accurate”
The principle of Nanocoulter Ⅰ determines that the measured data is real particle size information, without the need for inversion or derivation. It is equipped with a variety of known particle size and concentration of standard particles (60-300nm, 20nm per gradient) as the baseline data to achieve quantitative calibration of measured data to ensure that the particle size and concentration data are absolutely accurate. Both the mixed samples of different particle sizes and the samples of the same particle size with slight differences can be accurately identified.
.jpg)
Fig. 7 Minor differences in standard microspheres produced by different manufacturers
Nanocoulter Ⅰ is “firm”
Based on the classic Coulter principle, Nanocoulter Ⅰ has been working on micro-nano machining technology for more than 10 years. Its precision micro-machining capability is at atomic level (below 1 nanometer), with the highest resolution up to 1nm. Each particle passing through nanopore produces a potential pulse, so all exosome particles in the sample can be accurately measured to achieve single particle detection in the true sense. The real distribution of exosome particles in exosome samples can be seen intuitively during the test, and the report can be output immediately after completing the test.
.jpg)
Fig. 8 Signal pulse diagram of exosome sample detection
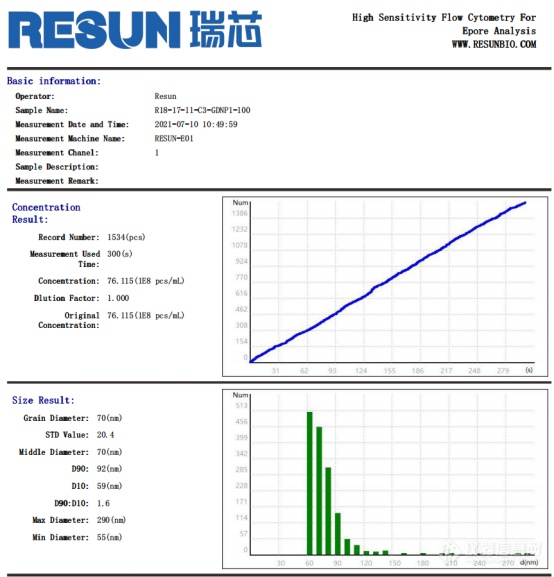
Fig. 9 Test report of exosome sample
In addition to the research on disease diagnosis, drug delivery and other medical aspects, exosomes have also been widely developed and utilized in the fields of beauty and health care. More and more scholars are engaged in the research on exosomes. Developed by Resun (Shenzhen) Technology Co., Ltd., Nanocoulter Ⅰ nano particle size analyzer will always be the helpful partner during research and development.
References:
Isolation and characterization of urinary extracellular vesicles: implications for biomarker discover. NATURE REVIEWS | NEPHROLOGY, 10.1038/nrneph.2017.148.
Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation,Nanomedicine: Nanotechnology, Biology and Medicine,2017.
Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing,Journal of Extracellular Vesicles,2018.
Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: yield, purity and functional potential,Journal of Extracellular Vesicles,2019.


